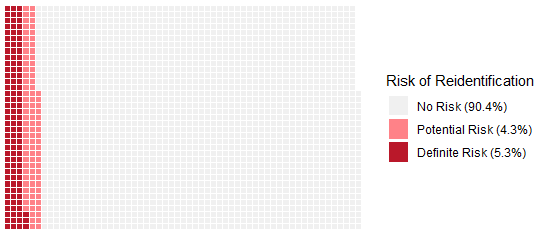We just published our findings and recommendations for researchers to better protect the privacy of research participants when sharing open data. Examining over 2,000 public datasets from papers using human participants, we found that 5-10% contained information that could potentially re-identify participants.